#ml 5.06
Photo




adrienette vs marichat, which will be angstier
#miraculous ladybug#mledit#adrien agreste#marinette dupain-cheng#kagami tsurugi#tikki#gifs#*#ml spoilers#ml 5.06
13K notes
·
View notes
Text





DETERMINATION
season 5, episode 6
#*#miraculous ladybug#ml#marinette dupain cheng#adrien agreste#kagami tsurugi#ml 5.06#ml spoilers#ml s5#adrinette#marigami#adrigami#mledit#miraculousedit
96 notes
·
View notes
Photo

FFCA Body Lotion Bleichcreme 50 Ml Aufhellende Bodylotion Die Sonne Für Bilder Preis : 5.06 € jetzt kaufen Artikelmerkmale Artikelzustand: Neu: Neuer, unbenutzter und unbeschädigter Artikel in nicht geöffneter Originalverpackung (soweit eine …
#body cream#body creme#body pflegecreme#Bodylotion#bodylotion männer#enthaarungscreme#enthaarungspads#enthaarungswachs#Körpercreme#Körperlotion#Körperpflege
0 notes
Text
A tweet
This is the Polarstern. When it locked itself in ice in October, Arctic sea extent was 5.06 ml,sq,km
Since then the Arctic has grown at 61,975 sq,km *per day to 14.68 ml,sq,km.
It’s the largest recovery of Arctic sea ice in satellite history(41yrs) pic.twitter.com/Oek11NV8AC
— Damo Pelham🦈 (@DamoPelham) March 9, 2020
0 notes
Text
crocetin could be considered as a promising anti-atherogenic candidate for future studies.
PMID: Food Funct. 2019 Nov 1 ;10(11):7461-7475. Epub 2019 Oct 31. PMID: 31667483 Abstract Title: The effect of crocetin supplementation on markers of atherogenic risk in patients with coronary artery disease: a pilot, randomized, double-blind, placebo-controlled clinical trial. Abstract: BACKGROUND AND PURPOSE: Molecular mechanisms of atherogenesis are considered to be emerging therapeutic targets for atherosclerosis prevention. Cell and animal studies have shown that crocetin can decelerate atherogenesis. However, the anti-atherogenic properties of crocetin in humans are still ambiguous.METHODS AND RESULTS: Fifty clinically diagnosed CAD patients were randomly divided into two parallel groups, crocetin and placebo, who received one capsule of crocetin (10 mg) and placebo per day, respectively, for two months. Serum circulating homocysteine (Hcy) [-1.09 (-1.64 to -0.54)μM, P = 0.001], heart-type fatty acid binding protein (h-FABP) [-2.07 (-2.72 to -1.43) ng mL, P = 0.001], intercellular adhesion molecule 1 [-14.92 (-21.92 to -7.92) ng mL, P = 0.001], vascular cell adhesion molecule 1 [-18.61 (-29.73 to -7.49) ng mL, P = 0.002], and monocyte chemoattractant protein 1 [-4.67 (-6.50 to -2.83) pg mL, P = 0.001] decreased significantly after the trial in the crocetin group, while high-density lipoprotein (HDL) significantly increased [+4.21 (0.68 to 7.73) mg mL, P = 0.021]. Also, systolic [-0.21 (-0.32 to -0.10) mmHg, P = 0.001] and diastolic [-0.20 (-0.34 to -0.07) mmHg, P = 0.004] blood pressures decreased significantly in the crocetin group. Nevertheless, clinically significant percentage changes were only observed in Hcy (-15.25± 3.15, μM), HDL (-10.70 ± 5.06, mg dL), and h-FABP (-21.10± 3.09, ng mL) in the crocetin group. Furthermore, the relative increase in the gene expressions of sirtuin1 and AMP-activated protein kinase and a decrease in the lectin-type oxidized LDL receptor 1 and nuclear factor-kappa B expression in isolated peripheral blood mononuclear cells in the crocetin group were significant at the end of the trial in comparison with the placebo.CONCLUSION: As the first human study, we showed the ability of crocetin to alter the expression of atherogenic genes and endothelial cell adhesion molecules in CAD patients. It appears that crocetin could be considered as a promising anti-atherogenic candidate for future studies.
read more
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION
Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms.
Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva
Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation.
Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9
Sublimation
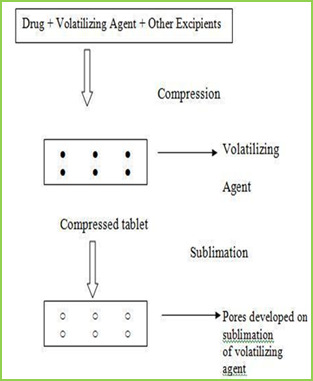
The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents,
MATERIAL & METHODS
Table no-1
Sr no.
Name of Ingredient
Supplier
1
Lamotrigine
Abottpharmapvt. Ltd., Goa
2
Sodium StarchGlycollate
Yash Scientific Enterprises, Pune
3
Crosscarmellose Sodium
Kurla Complex, Mumbai
4
β-Cyclodextrin
Ozone international, Mumbai
5
Aerosil
Yash Scientific Enterprises, Pune
6
Camphor
Yash Scientific Enterprises, Pune
7
Directly Compressible Lactose
Yash Scientific Enterprises, Pune
Method
Preformulation Study
Organoleptic Characteristics
Physico-chemical Characterization
1 Bulk Density
2 Tapped Density
3 Carr‘s index
4 Hausner‘s Ratio
5 Angle of Repose
Calibration curve of Drug
Formulation & Evaluation of Tablet
Hardness
Disintegration Time
Thickness
Friability
Wetting Time
Drug Content
Weight Variation
8. Invitro Drug Release (Dissolution Study)
Formulation procedure of tablet (direct compression)
In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant.
Table no-2
Sr No.
Name of
ingredients
F1 (mg)
F2 (mg)
F3 (mg)
F4 (mg)
F5 (mg)
F6 (mg)
1
Lamotrigine
25
25
25
25
25
25
2
β-cyclodextrin
25
25
25
25
25
25
3
Sodium starch
21
26.25
31.5
-
-
-
glycolate
4
Crosscarmellose
-
-
-
21
26.25
31.5
sodium
5
Direct
compressible
10.7
5.45
0.2
10.7
5.45
0.2
6
Camphor
7
7
7
7
7
7
8
Total weight
90
90
90
90
90
90
RESULTS AND DISCUSSION
In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated.
Organoleptic Characteristics
Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table
Table No.3 Organoleptic properties of Lamotrigine
Sr. No
Properties
Specification
Lamotrigine
1
Appearance
White
White
2
Description
Crystalline
Crystalline
3
Odour
Odourless
Odourless
4
Taste
Bitter
Bitter
Physical characterization
The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose.
Batch
code
Bulk density
(gm/ml) ± SD
Tapped density
(gm/ml) ± SD
Carr’s index
% ± SD
Hausner’s ratio
% ± SD
Angle of
repose (0) ± SD
F1
0.6032±0.03
0.6912 ± 0.01
14.25 ± 0.20
1.1124 ± 0.02
20.07 ± 0.54
F2
0.6133 ± 0.05
0.6999 ± 0.02
14.09 ± 0.39
1.1358 ± 0.07
19.45 ± 0.85
F3
0.6258 ± 0.01
0.7134 ± 0.06
15.00 ± 0.13
1.1425 ± 0.06
19.39 ± 0.29
F4
0.6078 ± 0.07
0.7088 ± 0.09
15.04 ± 0.75
1.1298 ± 0.04
20.14 ± 0.17
F5
0.6125 ± 0.02
0.7032 ± 0.05
14.58 ± 0.09
1.1340 ± 0.03
20.73 ± 0.65
F6
0.6289 ± 0.08
0.7155 ± 0.04
14.99 ± 0.67
1.1536 ± 0.01
20.10 ± 0.44
Calibration curve of Drug
Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table.
Table No 5 Calibration curve of lamotrigine in 0.1 N HCL
Sr. No.
Concentration (µg/ml)
Absorbance at 267 nm ±SD
1
0
0 ± 00
2
2
0.1927± 0.00015
3
4
0.2360± 0.00023
4
6
0.2924± 0.00011
5
8
0.3207± 0.00046
6
10
0.3913± 0.00078
Calibration curve
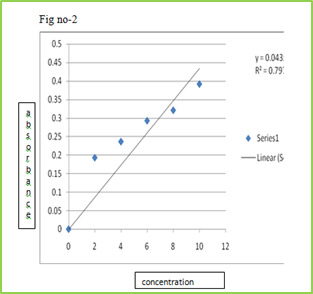
Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl
Evaluation of compression characteristics of formulations
Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table.
Tablet No.6 Post compression properties of tablets F1 to F6
Batch
code
Weight variation
(mg) ± SD
Hardness (kg/cm2)
± SD
Thickness (mm)
± SD
Friability ±
SD%
F1
0.090 ± 0.02
12.25 ± 0.28
5.02 ± 0.03
0.63 ± 0.02
F2
0.090 ± 0.01
13.20 ± 0.90
5.05 ± 0.08
0.52 ± 0.02
F3
0.090 ± 0.03
13.00 ± 0.26
5.06 ± 0.02
0.73 ± 0.01
F4
0.090 ± 0.02
13.10 ± 0.13
5.07 ± 0.05
0.89 ± 0.03
F5
0.090 ± 0.03
13.23 ± 0.58
5.03 ± 0.04
0.75 ± 0.04
F6
0.090 ± 0.01
13.05 ± 0.10
5.04 ± 0.01
0.45 ± 0.03
Evaluation of various Parameters of Tablets
The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet.
Table No.7 other post compression parameters of tablets F1 to F6
Batch
code
Disintegration
time (s)± SD
Wetting Time (s)
± SD
Drug content
± SD
F1
58.05 ± 0.07
62.37 ± 0.54
95.49 ± 1.11
F2
53.14 ± 0.04
59.48 ± 0.34
96.76 ± 0.92
F3
45.38 ± 0.03
56.35 ± 0.12
98.48 ± 1.07
F4
15.20 ± 0.10
57.01 ± 0.89
97.68 ± 1.15
F5
17.02 ± 0.07
55.42 ± 0.45
95.21 ± 1.01
F6
08.20 ± 0.03
53.32 ± 0.75
101.23 ± 1.05
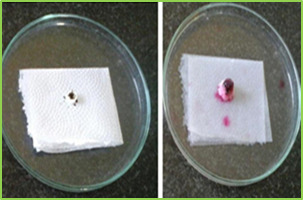
Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec)
In vitro Drug Release (Dissolution Study)
Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables.
Table No.7 In vitro drug release data of formulation F1 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0415
1.22
0.048
4.8
2
0.1737
5.18
0.20
20.43
5
0.3995
11.75
0.47
47.00
15
0.5805
17.07
0.68
68.28
30
0.8298
24.40
0.97
97.60
Table No.8 In vitro drug release data of formulation F2 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0761
2.23
0.08
8.9
2
0.1908
5.61
0.22
22.44
5
0.38.37
11.28
0.45
45.12
15
0.5638
16.58
0.66
66.32
30
0.8344
24.54
0.98
98.16
Table No.9 In vitro drug release data of formulation F3 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0625
1.83
0.07
7.35
2
0.2248
6.61
0.26
26.44
5
0.4158
12.22
0.48
48.88
15
0.5960
17.52
0.70
70.08
30
0.8495
24.98
0.99
99.92
Table No.10 In vitro drug release data of formulation F4 (n=2)
Time
(min)
Absor-bance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0305
0.89
0.03
3.5
2
0.2348
6.90
0.27
27.62
5
0.4009
11.79
0.47
47.16
15
0.6010
17.67
0.70
70.68
30
0.8489
24.96
0.99
99.84
Table No.11 in vitro drug release data of formulation F5 (n=2)
Time
(min)
Absorbance
(267nm)
Concen
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0238
0.7
0.02
2.8
2
0.1983
5.83
0.23
23.32
5
0.3785
11.13
0.44
44.52
15
0.5969
17.55
0.70
70.20
30
0.8239
24.23
0.96
96.92
Table No.12 In vitro drug release data of formulation F6 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0843
2.47
0.09
9.9
2
0.2248
6.61
0.26
26.44
5
0.4475
13.16
0.52
52.64
15
0.6308
18.55
0.74
74.20
30
0.8399
24.70
0.98
98.81
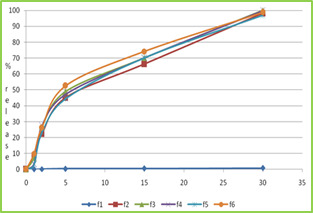
Figure no-4 dissolution profile of formulation F1 to F6
CONCLUSION
The results obtained so far encouraged as to derive following conclusion,
Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor.
The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture.
The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased.
Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces.
The post compression parameters of all formulations were determined and the values were found to be within IP limits.
In-vitro disintegration of F3 gives rapid disintegrating time and wetting time.
As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate.
The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics.
REFERENCES
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy.
Third Edition, Varghese Publication House, Bombay, India 1987: 296‐
Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets.
Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214
Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481
Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office.
Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995.
Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994.
Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028
Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office.
Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30.
Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42.
Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975.
Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279
Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448
Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University.
Read the full article
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION
Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms.
Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva
Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation.
Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9
Sublimation
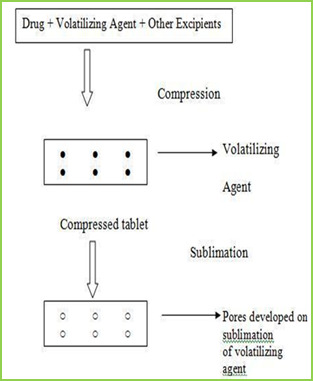
The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents,
MATERIAL & METHODS
Table no-1
Sr no.
Name of Ingredient
Supplier
1
Lamotrigine
Abottpharmapvt. Ltd., Goa
2
Sodium StarchGlycollate
Yash Scientific Enterprises, Pune
3
Crosscarmellose Sodium
Kurla Complex, Mumbai
4
β-Cyclodextrin
Ozone international, Mumbai
5
Aerosil
Yash Scientific Enterprises, Pune
6
Camphor
Yash Scientific Enterprises, Pune
7
Directly Compressible Lactose
Yash Scientific Enterprises, Pune
Method
Preformulation Study
Organoleptic Characteristics
Physico-chemical Characterization
1 Bulk Density
2 Tapped Density
3 Carr‘s index
4 Hausner‘s Ratio
5 Angle of Repose
Calibration curve of Drug
Formulation & Evaluation of Tablet
Hardness
Disintegration Time
Thickness
Friability
Wetting Time
Drug Content
Weight Variation
8. Invitro Drug Release (Dissolution Study)
Formulation procedure of tablet (direct compression)
In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant.
Table no-2
Sr No.
Name of
ingredients
F1 (mg)
F2 (mg)
F3 (mg)
F4 (mg)
F5 (mg)
F6 (mg)
1
Lamotrigine
25
25
25
25
25
25
2
β-cyclodextrin
25
25
25
25
25
25
3
Sodium starch
21
26.25
31.5
-
-
-
glycolate
4
Crosscarmellose
-
-
-
21
26.25
31.5
sodium
5
Direct
compressible
10.7
5.45
0.2
10.7
5.45
0.2
6
Camphor
7
7
7
7
7
7
8
Total weight
90
90
90
90
90
90
RESULTS AND DISCUSSION
In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated.
Organoleptic Characteristics
Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table
Table No.3 Organoleptic properties of Lamotrigine
Sr. No
Properties
Specification
Lamotrigine
1
Appearance
White
White
2
Description
Crystalline
Crystalline
3
Odour
Odourless
Odourless
4
Taste
Bitter
Bitter
Physical characterization
The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose.
Batch
code
Bulk density
(gm/ml) ± SD
Tapped density
(gm/ml) ± SD
Carr’s index
% ± SD
Hausner’s ratio
% ± SD
Angle of
repose (0) ± SD
F1
0.6032±0.03
0.6912 ± 0.01
14.25 ± 0.20
1.1124 ± 0.02
20.07 ± 0.54
F2
0.6133 ± 0.05
0.6999 ± 0.02
14.09 ± 0.39
1.1358 ± 0.07
19.45 ± 0.85
F3
0.6258 ± 0.01
0.7134 ± 0.06
15.00 ± 0.13
1.1425 ± 0.06
19.39 ± 0.29
F4
0.6078 ± 0.07
0.7088 ± 0.09
15.04 ± 0.75
1.1298 ± 0.04
20.14 ± 0.17
F5
0.6125 ± 0.02
0.7032 ± 0.05
14.58 ± 0.09
1.1340 ± 0.03
20.73 ± 0.65
F6
0.6289 ± 0.08
0.7155 ± 0.04
14.99 ± 0.67
1.1536 ± 0.01
20.10 ± 0.44
Calibration curve of Drug
Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table.
Table No 5 Calibration curve of lamotrigine in 0.1 N HCL
Sr. No.
Concentration (µg/ml)
Absorbance at 267 nm ±SD
1
0
0 ± 00
2
2
0.1927± 0.00015
3
4
0.2360± 0.00023
4
6
0.2924± 0.00011
5
8
0.3207± 0.00046
6
10
0.3913± 0.00078
Calibration curve
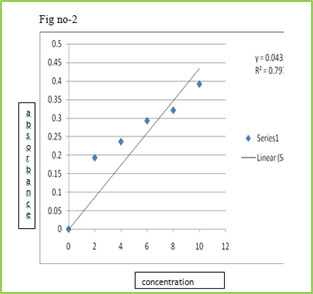
Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl
Evaluation of compression characteristics of formulations
Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table.
Tablet No.6 Post compression properties of tablets F1 to F6
Batch
code
Weight variation
(mg) ± SD
Hardness (kg/cm2)
± SD
Thickness (mm)
± SD
Friability ±
SD%
F1
0.090 ± 0.02
12.25 ± 0.28
5.02 ± 0.03
0.63 ± 0.02
F2
0.090 ± 0.01
13.20 ± 0.90
5.05 ± 0.08
0.52 ± 0.02
F3
0.090 ± 0.03
13.00 ± 0.26
5.06 ± 0.02
0.73 ± 0.01
F4
0.090 ± 0.02
13.10 ± 0.13
5.07 ± 0.05
0.89 ± 0.03
F5
0.090 ± 0.03
13.23 ± 0.58
5.03 ± 0.04
0.75 ± 0.04
F6
0.090 ± 0.01
13.05 ± 0.10
5.04 ± 0.01
0.45 ± 0.03
Evaluation of various Parameters of Tablets
The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet.
Table No.7 other post compression parameters of tablets F1 to F6
Batch
code
Disintegration
time (s)± SD
Wetting Time (s)
± SD
Drug content
± SD
F1
58.05 ± 0.07
62.37 ± 0.54
95.49 ± 1.11
F2
53.14 ± 0.04
59.48 ± 0.34
96.76 ± 0.92
F3
45.38 ± 0.03
56.35 ± 0.12
98.48 ± 1.07
F4
15.20 ± 0.10
57.01 ± 0.89
97.68 ± 1.15
F5
17.02 ± 0.07
55.42 ± 0.45
95.21 ± 1.01
F6
08.20 ± 0.03
53.32 ± 0.75
101.23 ± 1.05
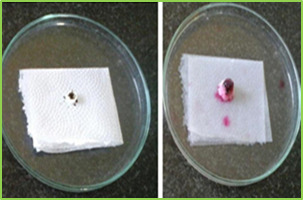
Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec)
In vitro Drug Release (Dissolution Study)
Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables.
Table No.7 In vitro drug release data of formulation F1 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0415
1.22
0.048
4.8
2
0.1737
5.18
0.20
20.43
5
0.3995
11.75
0.47
47.00
15
0.5805
17.07
0.68
68.28
30
0.8298
24.40
0.97
97.60
Table No.8 In vitro drug release data of formulation F2 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0761
2.23
0.08
8.9
2
0.1908
5.61
0.22
22.44
5
0.38.37
11.28
0.45
45.12
15
0.5638
16.58
0.66
66.32
30
0.8344
24.54
0.98
98.16
Table No.9 In vitro drug release data of formulation F3 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0625
1.83
0.07
7.35
2
0.2248
6.61
0.26
26.44
5
0.4158
12.22
0.48
48.88
15
0.5960
17.52
0.70
70.08
30
0.8495
24.98
0.99
99.92
Table No.10 In vitro drug release data of formulation F4 (n=2)
Time
(min)
Absor-bance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0305
0.89
0.03
3.5
2
0.2348
6.90
0.27
27.62
5
0.4009
11.79
0.47
47.16
15
0.6010
17.67
0.70
70.68
30
0.8489
24.96
0.99
99.84
Table No.11 in vitro drug release data of formulation F5 (n=2)
Time
(min)
Absorbance
(267nm)
Concen
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0238
0.7
0.02
2.8
2
0.1983
5.83
0.23
23.32
5
0.3785
11.13
0.44
44.52
15
0.5969
17.55
0.70
70.20
30
0.8239
24.23
0.96
96.92
Table No.12 In vitro drug release data of formulation F6 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0843
2.47
0.09
9.9
2
0.2248
6.61
0.26
26.44
5
0.4475
13.16
0.52
52.64
15
0.6308
18.55
0.74
74.20
30
0.8399
24.70
0.98
98.81
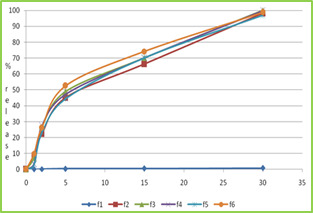
Figure no-4 dissolution profile of formulation F1 to F6
CONCLUSION
The results obtained so far encouraged as to derive following conclusion,
Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor.
The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture.
The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased.
Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces.
The post compression parameters of all formulations were determined and the values were found to be within IP limits.
In-vitro disintegration of F3 gives rapid disintegrating time and wetting time.
As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate.
The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics.
REFERENCES
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy.
Third Edition, Varghese Publication House, Bombay, India 1987: 296‐
Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets.
Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214
Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481
Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office.
Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995.
Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994.
Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028
Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office.
Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30.
Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42.
Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975.
Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279
Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448
Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University.
Read the full article
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION
Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms.
Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva
Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation.
Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9
Sublimation

The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents,
MATERIAL & METHODS
Table no-1
Sr no.
Name of Ingredient
Supplier
1
Lamotrigine
Abottpharmapvt. Ltd., Goa
2
Sodium StarchGlycollate
Yash Scientific Enterprises, Pune
3
Crosscarmellose Sodium
Kurla Complex, Mumbai
4
β-Cyclodextrin
Ozone international, Mumbai
5
Aerosil
Yash Scientific Enterprises, Pune
6
Camphor
Yash Scientific Enterprises, Pune
7
Directly Compressible Lactose
Yash Scientific Enterprises, Pune
Method
Preformulation Study
Organoleptic Characteristics
Physico-chemical Characterization
1 Bulk Density
2 Tapped Density
3 Carr‘s index
4 Hausner‘s Ratio
5 Angle of Repose
Calibration curve of Drug
Formulation & Evaluation of Tablet
Hardness
Disintegration Time
Thickness
Friability
Wetting Time
Drug Content
Weight Variation
8. Invitro Drug Release (Dissolution Study)
Formulation procedure of tablet (direct compression)
In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant.
Table no-2
Sr No.
Name of
ingredients
F1 (mg)
F2 (mg)
F3 (mg)
F4 (mg)
F5 (mg)
F6 (mg)
1
Lamotrigine
25
25
25
25
25
25
2
β-cyclodextrin
25
25
25
25
25
25
3
Sodium starch
21
26.25
31.5
-
-
-
glycolate
4
Crosscarmellose
-
-
-
21
26.25
31.5
sodium
5
Direct
compressible
10.7
5.45
0.2
10.7
5.45
0.2
6
Camphor
7
7
7
7
7
7
8
Total weight
90
90
90
90
90
90
RESULTS AND DISCUSSION
In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated.
Organoleptic Characteristics
Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table
Table No.3 Organoleptic properties of Lamotrigine
Sr. No
Properties
Specification
Lamotrigine
1
Appearance
White
White
2
Description
Crystalline
Crystalline
3
Odour
Odourless
Odourless
4
Taste
Bitter
Bitter
Physical characterization
The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose.
Batch
code
Bulk density
(gm/ml) ± SD
Tapped density
(gm/ml) ± SD
Carr’s index
% ± SD
Hausner’s ratio
% ± SD
Angle of
repose (0) ± SD
F1
0.6032±0.03
0.6912 ± 0.01
14.25 ± 0.20
1.1124 ± 0.02
20.07 ± 0.54
F2
0.6133 ± 0.05
0.6999 ± 0.02
14.09 ± 0.39
1.1358 ± 0.07
19.45 ± 0.85
F3
0.6258 ± 0.01
0.7134 ± 0.06
15.00 ± 0.13
1.1425 ± 0.06
19.39 ± 0.29
F4
0.6078 ± 0.07
0.7088 ± 0.09
15.04 ± 0.75
1.1298 ± 0.04
20.14 ± 0.17
F5
0.6125 ± 0.02
0.7032 ± 0.05
14.58 ± 0.09
1.1340 ± 0.03
20.73 ± 0.65
F6
0.6289 ± 0.08
0.7155 ± 0.04
14.99 ± 0.67
1.1536 ± 0.01
20.10 ± 0.44
Calibration curve of Drug
Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table.
Table No 5 Calibration curve of lamotrigine in 0.1 N HCL
Sr. No.
Concentration (µg/ml)
Absorbance at 267 nm ±SD
1
0
0 ± 00
2
2
0.1927± 0.00015
3
4
0.2360± 0.00023
4
6
0.2924± 0.00011
5
8
0.3207± 0.00046
6
10
0.3913± 0.00078
Calibration curve

Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl
Evaluation of compression characteristics of formulations
Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table.
Tablet No.6 Post compression properties of tablets F1 to F6
Batch
code
Weight variation
(mg) ± SD
Hardness (kg/cm2)
± SD
Thickness (mm)
± SD
Friability ±
SD%
F1
0.090 ± 0.02
12.25 ± 0.28
5.02 ± 0.03
0.63 ± 0.02
F2
0.090 ± 0.01
13.20 ± 0.90
5.05 ± 0.08
0.52 ± 0.02
F3
0.090 ± 0.03
13.00 ± 0.26
5.06 ± 0.02
0.73 ± 0.01
F4
0.090 ± 0.02
13.10 ± 0.13
5.07 ± 0.05
0.89 ± 0.03
F5
0.090 ± 0.03
13.23 ± 0.58
5.03 ± 0.04
0.75 ± 0.04
F6
0.090 ± 0.01
13.05 ± 0.10
5.04 ± 0.01
0.45 ± 0.03
Evaluation of various Parameters of Tablets
The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet.
Table No.7 other post compression parameters of tablets F1 to F6
Batch
code
Disintegration
time (s)± SD
Wetting Time (s)
± SD
Drug content
± SD
F1
58.05 ± 0.07
62.37 ± 0.54
95.49 ± 1.11
F2
53.14 ± 0.04
59.48 ± 0.34
96.76 ± 0.92
F3
45.38 ± 0.03
56.35 ± 0.12
98.48 ± 1.07
F4
15.20 ± 0.10
57.01 ± 0.89
97.68 ± 1.15
F5
17.02 ± 0.07
55.42 ± 0.45
95.21 ± 1.01
F6
08.20 ± 0.03
53.32 ± 0.75
101.23 ± 1.05

Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec)
In vitro Drug Release (Dissolution Study)
Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables.
Table No.7 In vitro drug release data of formulation F1 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0415
1.22
0.048
4.8
2
0.1737
5.18
0.20
20.43
5
0.3995
11.75
0.47
47.00
15
0.5805
17.07
0.68
68.28
30
0.8298
24.40
0.97
97.60
Table No.8 In vitro drug release data of formulation F2 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0761
2.23
0.08
8.9
2
0.1908
5.61
0.22
22.44
5
0.38.37
11.28
0.45
45.12
15
0.5638
16.58
0.66
66.32
30
0.8344
24.54
0.98
98.16
Table No.9 In vitro drug release data of formulation F3 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0625
1.83
0.07
7.35
2
0.2248
6.61
0.26
26.44
5
0.4158
12.22
0.48
48.88
15
0.5960
17.52
0.70
70.08
30
0.8495
24.98
0.99
99.92
Table No.10 In vitro drug release data of formulation F4 (n=2)
Time
(min)
Absor-bance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0305
0.89
0.03
3.5
2
0.2348
6.90
0.27
27.62
5
0.4009
11.79
0.47
47.16
15
0.6010
17.67
0.70
70.68
30
0.8489
24.96
0.99
99.84
Table No.11 in vitro drug release data of formulation F5 (n=2)
Time
(min)
Absorbance
(267nm)
Concen
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0238
0.7
0.02
2.8
2
0.1983
5.83
0.23
23.32
5
0.3785
11.13
0.44
44.52
15
0.5969
17.55
0.70
70.20
30
0.8239
24.23
0.96
96.92
Table No.12 In vitro drug release data of formulation F6 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0843
2.47
0.09
9.9
2
0.2248
6.61
0.26
26.44
5
0.4475
13.16
0.52
52.64
15
0.6308
18.55
0.74
74.20
30
0.8399
24.70
0.98
98.81

Figure no-4 dissolution profile of formulation F1 to F6
CONCLUSION
The results obtained so far encouraged as to derive following conclusion,
Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor.
The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture.
The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased.
Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces.
The post compression parameters of all formulations were determined and the values were found to be within IP limits.
In-vitro disintegration of F3 gives rapid disintegrating time and wetting time.
As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate.
The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics.
REFERENCES
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy.
Third Edition, Varghese Publication House, Bombay, India 1987: 296‐
Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets.
Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214
Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481
Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office.
Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995.
Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994.
Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028
Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office.
Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30.
Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42.
Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975.
Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279
Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448
Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University.
Read the full article
0 notes
Photo




#miraculous ladybug#mledit#marichat#tikki#marinette dupain-cheng#chat noir#cat noir#ml#gifs#*#ml spoilers#ml 5.06
8K notes
·
View notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION
Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms.
Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva
Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation.
Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9
Sublimation

The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents,
MATERIAL & METHODS
Table no-1
Sr no.
Name of Ingredient
Supplier
1
Lamotrigine
Abottpharmapvt. Ltd., Goa
2
Sodium StarchGlycollate
Yash Scientific Enterprises, Pune
3
Crosscarmellose Sodium
Kurla Complex, Mumbai
4
β-Cyclodextrin
Ozone international, Mumbai
5
Aerosil
Yash Scientific Enterprises, Pune
6
Camphor
Yash Scientific Enterprises, Pune
7
Directly Compressible Lactose
Yash Scientific Enterprises, Pune
Method
Preformulation Study
Organoleptic Characteristics
Physico-chemical Characterization
1 Bulk Density
2 Tapped Density
3 Carr‘s index
4 Hausner‘s Ratio
5 Angle of Repose
Calibration curve of Drug
Formulation & Evaluation of Tablet
Hardness
Disintegration Time
Thickness
Friability
Wetting Time
Drug Content
Weight Variation
8. Invitro Drug Release (Dissolution Study)
Formulation procedure of tablet (direct compression)
In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant.
Table no-2
Sr No.
Name of
ingredients
F1 (mg)
F2 (mg)
F3 (mg)
F4 (mg)
F5 (mg)
F6 (mg)
1
Lamotrigine
25
25
25
25
25
25
2
β-cyclodextrin
25
25
25
25
25
25
3
Sodium starch
21
26.25
31.5
-
-
-
glycolate
4
Crosscarmellose
-
-
-
21
26.25
31.5
sodium
5
Direct
compressible
10.7
5.45
0.2
10.7
5.45
0.2
6
Camphor
7
7
7
7
7
7
8
Total weight
90
90
90
90
90
90
RESULTS AND DISCUSSION
In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated.
Organoleptic Characteristics
Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table
Table No.3 Organoleptic properties of Lamotrigine
Sr. No
Properties
Specification
Lamotrigine
1
Appearance
White
White
2
Description
Crystalline
Crystalline
3
Odour
Odourless
Odourless
4
Taste
Bitter
Bitter
Physical characterization
The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose.
Batch
code
Bulk density
(gm/ml) ± SD
Tapped density
(gm/ml) ± SD
Carr’s index
% ± SD
Hausner’s ratio
% ± SD
Angle of
repose (0) ± SD
F1
0.6032±0.03
0.6912 ± 0.01
14.25 ± 0.20
1.1124 ± 0.02
20.07 ± 0.54
F2
0.6133 ± 0.05
0.6999 ± 0.02
14.09 ± 0.39
1.1358 ± 0.07
19.45 ± 0.85
F3
0.6258 ± 0.01
0.7134 ± 0.06
15.00 ± 0.13
1.1425 ± 0.06
19.39 ± 0.29
F4
0.6078 ± 0.07
0.7088 ± 0.09
15.04 ± 0.75
1.1298 ± 0.04
20.14 ± 0.17
F5
0.6125 ± 0.02
0.7032 ± 0.05
14.58 ± 0.09
1.1340 ± 0.03
20.73 ± 0.65
F6
0.6289 ± 0.08
0.7155 ± 0.04
14.99 ± 0.67
1.1536 ± 0.01
20.10 ± 0.44
Calibration curve of Drug
Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table.
Table No 5 Calibration curve of lamotrigine in 0.1 N HCL
Sr. No.
Concentration (µg/ml)
Absorbance at 267 nm ±SD
1
0
0 ± 00
2
2
0.1927± 0.00015
3
4
0.2360± 0.00023
4
6
0.2924± 0.00011
5
8
0.3207± 0.00046
6
10
0.3913± 0.00078
Calibration curve

Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl
Evaluation of compression characteristics of formulations
Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table.
Tablet No.6 Post compression properties of tablets F1 to F6
Batch
code
Weight variation
(mg) ± SD
Hardness (kg/cm2)
± SD
Thickness (mm)
± SD
Friability ±
SD%
F1
0.090 ± 0.02
12.25 ± 0.28
5.02 ± 0.03
0.63 ± 0.02
F2
0.090 ± 0.01
13.20 ± 0.90
5.05 ± 0.08
0.52 ± 0.02
F3
0.090 ± 0.03
13.00 ± 0.26
5.06 ± 0.02
0.73 ± 0.01
F4
0.090 ± 0.02
13.10 ± 0.13
5.07 ± 0.05
0.89 ± 0.03
F5
0.090 ± 0.03
13.23 ± 0.58
5.03 ± 0.04
0.75 ± 0.04
F6
0.090 ± 0.01
13.05 ± 0.10
5.04 ± 0.01
0.45 ± 0.03
Evaluation of various Parameters of Tablets
The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet.
Table No.7 other post compression parameters of tablets F1 to F6
Batch
code
Disintegration
time (s)± SD
Wetting Time (s)
± SD
Drug content
± SD
F1
58.05 ± 0.07
62.37 ± 0.54
95.49 ± 1.11
F2
53.14 ± 0.04
59.48 ± 0.34
96.76 ± 0.92
F3
45.38 ± 0.03
56.35 ± 0.12
98.48 ± 1.07
F4
15.20 ± 0.10
57.01 ± 0.89
97.68 ± 1.15
F5
17.02 ± 0.07
55.42 ± 0.45
95.21 ± 1.01
F6
08.20 ± 0.03
53.32 ± 0.75
101.23 ± 1.05

Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec)
In vitro Drug Release (Dissolution Study)
Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables.
Table No.7 In vitro drug release data of formulation F1 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0415
1.22
0.048
4.8
2
0.1737
5.18
0.20
20.43
5
0.3995
11.75
0.47
47.00
15
0.5805
17.07
0.68
68.28
30
0.8298
24.40
0.97
97.60
Table No.8 In vitro drug release data of formulation F2 (n=2)
Time
(min)
Absorbance
(267nm)
Concentration
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0761
2.23
0.08
8.9
2
0.1908
5.61
0.22
22.44
5
0.38.37
11.28
0.45
45.12
15
0.5638
16.58
0.66
66.32
30
0.8344
24.54
0.98
98.16
Table No.9 In vitro drug release data of formulation F3 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0625
1.83
0.07
7.35
2
0.2248
6.61
0.26
26.44
5
0.4158
12.22
0.48
48.88
15
0.5960
17.52
0.70
70.08
30
0.8495
24.98
0.99
99.92
Table No.10 In vitro drug release data of formulation F4 (n=2)
Time
(min)
Absor-bance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0305
0.89
0.03
3.5
2
0.2348
6.90
0.27
27.62
5
0.4009
11.79
0.47
47.16
15
0.6010
17.67
0.70
70.68
30
0.8489
24.96
0.99
99.84
Table No.11 in vitro drug release data of formulation F5 (n=2)
Time
(min)
Absorbance
(267nm)
Concen
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0238
0.7
0.02
2.8
2
0.1983
5.83
0.23
23.32
5
0.3785
11.13
0.44
44.52
15
0.5969
17.55
0.70
70.20
30
0.8239
24.23
0.96
96.92
Table No.12 In vitro drug release data of formulation F6 (n=2)
Time
(min)
Absorb-ance
(267nm)
Concn
(µg/ml)
Cumulative
drug release
Percentage
CDR (%)
0
0
0
0
0
1
0.0843
2.47
0.09
9.9
2
0.2248
6.61
0.26
26.44
5
0.4475
13.16
0.52
52.64
15
0.6308
18.55
0.74
74.20
30
0.8399
24.70
0.98
98.81

Figure no-4 dissolution profile of formulation F1 to F6
CONCLUSION
The results obtained so far encouraged as to derive following conclusion,
Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor.
The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture.
The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased.
Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces.
The post compression parameters of all formulations were determined and the values were found to be within IP limits.
In-vitro disintegration of F3 gives rapid disintegrating time and wetting time.
As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate.
The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics.
REFERENCES
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy.
Third Edition, Varghese Publication House, Bombay, India 1987: 296‐
Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets.
Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214
Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481
Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office.
Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995.
Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994.
Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028
Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office.
Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30.
Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42.
Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975.
Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279
Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448
Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University.
Read the full article
0 notes
Text
D8D9 Whitening Emulsion Body Lotion 50 Ml Die Sonne Bleichcreme Für Bilder
D8D9 Whitening Emulsion Body Lotion 50 Ml Die Sonne Bleichcreme Für Bilder

Preis : 5.06 €
jetzt kaufen
Artikelmerkmale Artikelzustand: Neu: Neuer, unbenutzter und unbeschädigter Artikel in nicht geöffneter Originalverpackung (soweit eine
Marke: Markenlos EAN: Nicht…
View On WordPress
#body cream#body creme#body pflegecreme#Bodylotion#bodylotion männer#enthaarungscreme#enthaarungspads#enthaarungswachs#Körpercreme#Körperlotion#Körperpflege
0 notes
Photo




#miraculous ladybug#mledit#marinette dupain-cheng#adrien agreste#luka couffaine#kagami tsurugi#adrienette#gifs#*#ml#ml spoilers#ml 5.06
7K notes
·
View notes
Photo









6K notes
·
View notes
Photo






#miraculous ladybug#meldit#adrien agreste#marinette dupain-cheng#adrienette#luka couffaine#lukanette#kagami tsurugi#gifs#*#ml#ml spoilers#ml 5.06
4K notes
·
View notes
Photo










4K notes
·
View notes
Photo





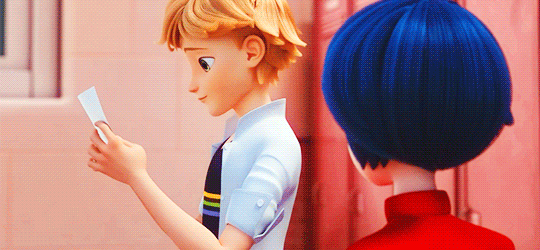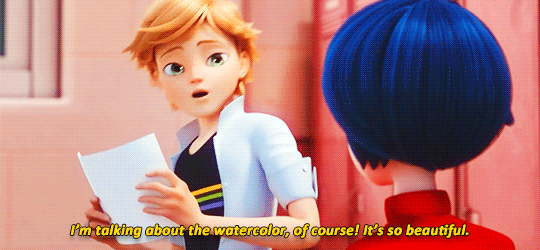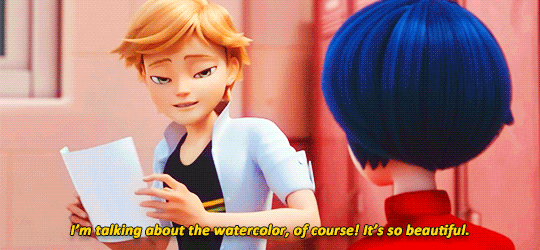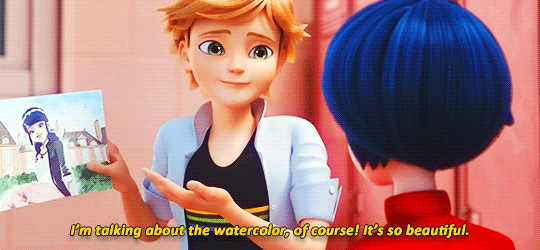
#miraculous ladybug#mledit#miraculousedit#kagami tsurugi#adrien agreste#adrienette#gifs#*#ml spoilers#ml 5.06
4K notes
·
View notes